The United States Food and Drug Administration says Johnson & Johnson’s one-shot vaccine is safe and protects against severe COVID-19 disease.
The agency released documents Wednesday in preparation for a meeting Friday to decide if it will approve the vaccine for emergency use.
The Johnson & Johnson, or J&J, large Phase 3 trials involved almost 44,000 volunteers. The vaccine appeared to work best in the United States with an efficacy rate of 72 percent. In Brazil the rate was 68 percent. And in South Africa, where a fast-spreading coronavirus variant was first identified last October, the rate was 64 percent effective. That is up from the 57 percent reported earlier by the drug maker.
The FDA also examined the vaccine in connection with coronavirus variants, especially those found in Brazil and South Africa. The independent scientists found that 28 days after vaccination the efficacy rate increases to as high as 87 percent.
Volunteers in the trial reported only minor side effects from the shots such as pain, fever and headache. By early February, there was no COVID-related deaths in the group of volunteers receiving the vaccine. There were seven deaths in the study group that received a placebo, a shot filled with an inactive substance.
The J&J vaccine can be stored at normal refrigeration temperature easing distribution operations. The two-shot vaccines from Pfizer-BioNTech and Moderna, already approved for emergency use, require extreme cold storage.
J&J told Congress at Tuesday’s hearing that it expected to provide 20 million shots by the end of March and 100 million by summer. Worldwide, the company said it aims to produce around one billion vaccines by the end of the year.
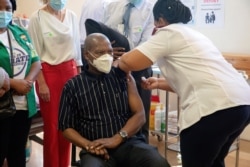
In other vaccine news, Ghana received the first transport of coronavirus vaccine from the United Nations-backed COVAX program on Wednesday.
The organization said it shipped 600,000 treatments of the Oxford-AstraZeneca vaccine to the African country. It expects to send some to the Ivory Coast later this week. And COVAX hopes to provide at least 2 billion shots to other poor countries.
I’m Caty Weaver.
Hai Do wrote this story for Learning English. Caty Weaver was the editor.
____________________________________________________________
Words in This Story
refrigeration - n. the process of keeping food, drink or medicine cold in order to preserve it
distribution - n. the act of delivering something to people or places