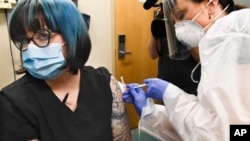
The world’s biggest COVID-19 vaccine study started on Monday. A total of 30,000 planned volunteers will help test the effectiveness and safety of a shot developed by the National Institutes of Health and drug maker Moderna.
The volunteers will each receive two doses of a shot. They will not know whether they are getting the real vaccine or a false version. Scientists will then closely follow the volunteers as they go about their daily activities. They want to see which group experiences a higher rate of infections, especially in areas where the virus still is spreading unchecked.
There is still no guarantee that the experimental vaccine will offer protection. The study hopes to answer that question.
Dr. Anthony Fauci is the nation's top infectious disease expert with NIH. He told the Associated Press, “Unfortunately for the United States of America, we have plenty of infections right now” to help get that answer.
Volunteers from more than 80 test areas across the country will take part in the study. Moderna said the first vaccines were given Monday morning in Savannah, Georgia.
Several other vaccine candidates made by China and Britain’s Oxford University started later-stage studies earlier this month. Those studies involved fewer volunteers than the U.S. ones. They are being carried out in Brazil and other hard-hit countries.
The U.S. government requires its own tests of any vaccine that might be used in the country. The aim is not just to test if a vaccine works. It is also to check if it is safe.
Through the government-funded COVID-19 Prevention Network, the U.S. plans a new study for vaccine candidates each month through autumn. Each will involve 30,000 newly chosen volunteers. The hope is that, by using the same rules for each study, scientists will be easily able to compare the vaccines.
In August, the U.S. will carry out its final stages of Oxford University’s vaccine candidate. The study of Johnson & Johnson’s vaccine will start in September and the one from Novavax in October. Drug maker Pfizer also plans to carry out its own 30,000-person study this summer.
That is a lot of volunteers needed to test possible vaccines. But in recent weeks, more than 150,000 Americans have registered to volunteer for the studies, says Dr. Larry Corey. He is with the Fred Hutchinson Cancer Research Institute in Seattle, Washington.
“These trials… need to reflect the diversity of the United States population,” Corey said at a vaccine meeting last week.
He said it is important that the studies include people from different age groups and from populations that have been hit especially hard by COVID-19, including Blacks and Hispanics.
It normally takes years to create a new vaccine from start to finish. But this time, scientists are setting speed records. They believe vaccination is the world’s best hope against the virus.
The new coronavirus was not even known to exist before late December. Vaccine makers got to work on January 10, when China shared the virus’ genetic sequence.
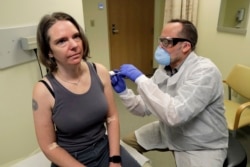
Just 65 days later, U.S. researchers gave the first test vaccine made by the NIH and Moderna to Jennifer Haller, a volunteer in Seattle, Washington. Haller is urging others to volunteer now.
She told the AP, “We all feel so helpless right now. There’s very little that we can do to combat this virus. And being able to participate in this trial has given me a sense of, that I’m doing something.”
That early study included Haller and 44 others. It showed the experimental COVID-19 vaccine produced antibodies in a small group of healthy people. It caused some minor side effects such as a brief fever, chills and pain at the injection area. Early tests of other leading vaccine candidates have had similarly promising results.
It will take several months for the first data to come in from the 30,000-volunteer test from Moderna, followed by the one from Oxford.
Until then, Haller will continue to wear a face covering in public. She is still following the same physical distancing advised for everyone. “I don’t know what the chances are that this is the exact right vaccine,” she said. “But thank goodness that there are so many others out there battling this right now.”
I'm Ashley Thompson.
Hai Do adapted this story for Learning English from an Associated Press news report. Ashley Thompson was the editor.
_______________________________________________________________
Words in This Story
dose - n. the amount of medicine that is taken at one time
stage - n. a particular period in the development of something
reflect - v. to show something
fever - n. a body temperature that is higher than normal
chill - n. a cold feeling